In the field of cell therapy for cardiovascular diseases, researchers see two main ways that the cells can provide benefits:
*As building blocks – actually replacing dead cells in damaged tissues
*As nurses — supplying growth factors and other supportive signals, but not becoming part of damaged tissues
Tension between these two roles arises partly from the source of the cells.
Many clinical trials have used bone marrow-derived cells, and the benefits here appear to come mostly from the “paracrine” nurse function. A more ambitious approach is to use progenitor-type cells, which may have to come from iPS cells or cardiac stem cells isolated via biopsy-like procedures. These cells may have a better chance of actually becoming part of the damaged tissue’s muscles or blood vessels, but they are more difficult to obtain and engineer.
A related concern: available evidence suggests introduced cells – no matter if they are primarily serving as nurses or building blocks — don’t survive or even stay in their target tissue for long.
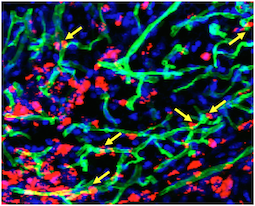
Transplanted cells were labeled with a red dye, while a perfused green dye shows the extent of functional blood vessels. Blue is DAPI, staining nuclear DNA. Yellow arrows indicate where red cells appear to contribute to green blood vessels. Courtesy of Sangho Lee.
Stem cell biologist Young-sup Yoon and colleagues recently published a paper in Biomaterials in which the authors use chitosan, a gel-like carbohydrate material obtained by processing crustacean shells, to aid in cell retention and survival. Ravi Bellamkonda’s lab at Georgia Tech contributed to the paper.
More refinement of these approaches are necessary before clinical use, Â but it illustrates how engineered mixtures of progenitor cells and supportive materials are becoming increasingly sophisticated and complicated.
The chitosan gel resembles the alginate material used to encapsulate cells by the Taylor lab. Yoon’s team was testing efficacy in a hindlimb ischemia model, in which a mouse’s leg is deprived of blood. This situation is analogous to peripheral artery disease, and the readout of success is the ability of experimental treatments to regrow capillaries in the damaged leg.
The current paper builds a bridge between the nurse and building block approaches, because the researchers mix two complementary types of cells: an angiogenic one derived from bone marrow cells that expands existing blood vessels, and a vasculogenic one derived from embryonic stem cells that drives formation of new blood vessels. Note: embryonic stem cells were of mouse origin, not human. Read more





