If you hear someone talking about a stress hormone, they’re probably talking about cortisol. It’s released by the adrenal glands in stressful situations, whether you have to escape a bear or just give a speech. Cortisol is supposed to prepare the body for “fight or flight.â€

Kerry Ressler, MD, PhD
Let’s step back a bit, and look at how the brain triggers cortisol production: through a peptide produced in the brain called CRF (corticotropin-releasing factor). CRF is elevated in several disorders such as depression and PTSD, and is also thought to be involved in drug and alcohol dependency.
Neurons that make CRF are found in locations all over the brain, so studying them can be tricky. Kerry Ressler and his colleagues have developed an intriguing tool for studying CRF. In the places where CRF is produced in a mouse’s brain, they can take out the gene of their choice.
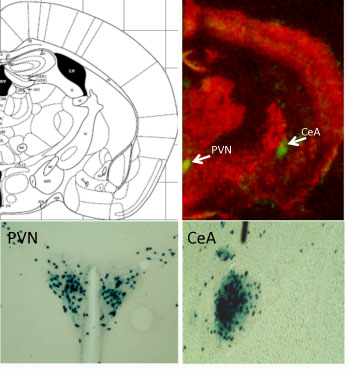
Green spots (above) and blue staining (below) indicate where CRF is produced in the mouse brain.
PVN = hypothalamus, paraventricular nucleus
CeA = central amygdala
In a new paper in PNAS, postdoc Georgette Gafford and Ressler use this tool in a subtle way. They have mice where a gene for a GABA receptor, one of the main inhibitory receptors (brakes) in the nervous system, is deleted, but only in the CRF neurons. This basically has the effect of turning up the volume on CRF production in several parts of the brain. It appears that modulating GABA receptors is something that normally happens to regulate CRF production, but in this case, a restraint on these stress-sensitive cells has been taken off.
“These mice are normal in many ways – normal locomotor and pain responses and no difference in depressive-like behavior or Pavlovian fear conditioning. However, these mutants have increased anxiety-like behavior,†Gafford and Ressler write.
They also have “impaired extinction of conditioned fear,†meaning that they have trouble becoming NOT afraid of something, like a buzzing sound, to which they have been sensitized by shocks. This is analogous to PTSD in which patients remain afraid and aren’t able to successfully inhibit their prior fear learning, even after the context is now safe. [A 2011 paper goes into more detail on this biological aspect of PTSD in a civilian population.]
“These data indicate that disturbance of this specific population of neurons causes increased anxiety and impaired fear extinction, and helps us to further understand mechanisms of fear- and anxiety-related disorders such as PTSD,” Ressler and Gafford write.
In the mutant mice, a drug that blocks CRF rescued their behavioral impairments. Some other recent investigations of mice with CRF overproduction in the brain revealed “surprising paradoxical effects.”
Drugs that block CRF have been in clinical trials, some with mixed results. A trial now proceeding at Emory is evaluating a CRF antagonist in women with PTSD.
Ressler, associate professor of psychiatry and behavioral sciences, is a Howard Hughes Medical Investigator, with a laboratory at the Yerkes National Primate Research Center. He is also co-director of the Grady Trauma Project.