 Raise your hand if you played tetherball in grade school. Paul Spearman and his colleagues have a new paper in the journal Cell Host & Microbe probing a protein called “tetherin†that keeps HIV ensnared within cells it is infecting.
Raise your hand if you played tetherball in grade school. Paul Spearman and his colleagues have a new paper in the journal Cell Host & Microbe probing a protein called “tetherin†that keeps HIV ensnared within cells it is infecting.
The paper includes electron microscopy images that make it possible to imagine a tiny cord attached to a nascent HIV particle within the cell. In these images, we don’t see the tetherin protein directly. However, we do see gold beads, bound to antibodies against the tetherin protein, which indicate where the protein is. The microscopy was performed at Emory’s Robert P. Apkarian Integrated Electron Microscopy Core.
Tetherin is a so-called “restriction factor,†one of several proteins within the cell that interfere with parts of the viral life
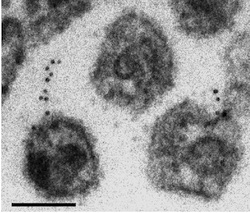
The black dots are antibody-linked gold beads, which indicate where the tetherin is. The larger globules are viral capsids.
cycle. Other restriction factors include enzymes that strip the viral RNA or impede the assembly of the viral capsid. Tetherin also interferes with a variety of other viruses such as Ebola.
Some viral proteins such as HIV’s Vpu or Nef fight back against the action of tetherin. Tracking how this kind of arms race has developed can help scientists follow how HIV evolved from similar retroviruses that infect non-human primates. In addition, knowing how tetherin works could be important in efforts to eradicate potential reservoirs of HIV in infected individuals, and in understanding how the virus is transmitted from person to person.
In their paper, first author Hin Chu and Spearman wanted to determine why infection looks different in two different cell types vulnerable to HIV. In T cells, HIV assembly occurs near the membrane, but in macrophages, HIV assembly occurs in an internal compartment.
“The reason that there is a large, internal collection of HIV particles in macrophages is hotly debated,†Spearman explains. “Some see this as a reservoir of virus that is available to spread to other cells, others would say this is a dead-end compartment. We found that the compartment basically goes away when we deplete tetherin, so tetherin is essential to the existence of the virus-containing compartment.â€
Chu and his co-workers examined what happened in macrophages when they used a tool called “RNA interference†to turn off the tetherin gene.

Hin Chu
“We found that cell-cell transmission was enhanced when we depleted tetherin. My interpretation is that when tetherin is upregulated in macrophages, viral particles are rapidly internalized and are not transmitted.â€
“Another significant finding is that Vpu doesn’t work well in macrophages. If we can determine why it doesn’t work well in this cell type, it will help us understand how Vpu does work so efficiently in other cells such as T cells. Macrophages are one of the most important cell types infected by HIV, so these questions are likely to be very important in how virus spreads and is maintained in infected individuals.â€
Spearman is chief research officer for Children’s Healthcare of Atlanta and director of the Children’s Center for Vaccines and Immunology, within the Emory-Children’s Pediatric Research Center. He is also professor and vice chair of research in pediatrics at Emory. Hin Chu is a graduate student in the Microbiology and Molecular Genetics program.





