These days, it sounds a bit old-fashioned to ask the question: “Where is consciousness located in the brain?†The prevailing thinking is that consciousness lives in the network, rather than in one particular place. Still, neuroscientists sometimes get an intriguing glimpse of a critical link in the network.
A recent paper in the journal Epilepsy & Behavior describes an epilepsy patient who had electrodes implanted within her brain at Emory University Hospital, because neurologists wanted to understand where her seizures were coming from and plan possible surgery. Medication had not controlled her seizures and previous surgery elsewhere had not either.
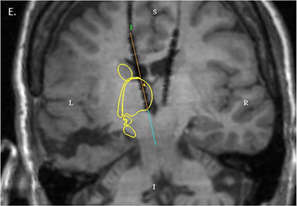
MRI showing electrode placement. Yellow outline indicates the location of the caudate and thalamus. Image from Leeman-Markowsi et al, Epilepsy & Behavior (2015).
During intracranial EEG monitoring, implanted electrodes detected a pattern of signals coming from one part of the thalamus, a central region of the brain. The pattern was present when the patient was conscious, and then stopped as soon as seizure activity made her lose awareness.
The pattern of signals had a characteristic frequency – around 35 times per second – so it helps to think of the signal as an auditory tone. Lead author Beth Leeman-Markowski, director of EUH’s Epilepsy Monitoring Unit at the time when the patient was evaluated, describes the signal as a “buzz.â€
“That buzz has something to do with maintenance of consciousness,†she says.
Previous studies have found that this part of the thalamus, the dorsomedial nucleus, participates in “attention, executive function, memory, orientation and arousal.†Neuroscientists have identified several regions of the brain that, like the DM thalamus, appear to be important for regulating awareness or being awake. A 2014 paper in Epilepsy & Behavior reports how electrical stimulation of the left claustrum/anterior insula caused an epilepsy patient at GWU to lose consciousness, without a seizure, for as long as the stimulation occurred.
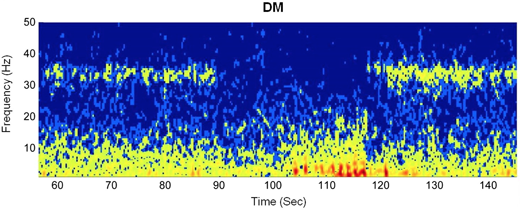
Intracranial EEG during a seizure captured signals from the dorsomedial nucleus of the thalamus (DM). The patient loses awareness at approximately 90 seconds and regains awareness at 120 seconds. The “buzz” is the fuzzy band between 30 and 40 Hz.
In the Emory patient, a simple on-off relationship held true. In more than 40 seizures that occurred while she was awake, the buzz from the DM thalamus was on during consciousness, off when a seizure interfered with consciousness, and then on again when the patient regained awareness. In contrast, the buzz was weaker and the on-off relationship during seizures was not as consistent when the patient was asleep. During seizures, the patient’s head would slump to the left and her left arm would flex, the paper describes.
Regarding the buzz, it didn’t make much difference whether the patient was eating, talking with family or watching television, Leeman-Markowski explains. During all those activities, the buzz was on. The main difference came from the patient’s being awake or asleep.
Leeman-Markowski stresses that seizure activity doesn’t appear to envelop the DM thalamus itself. Instead, seizures affected the activity of the midcingulate cortex, which has plentiful connections with the DM thalamus. When the seizure’s storm overwhelms the midcingulate cortex, the buzz from the DM thalamus stops.
At the end of monitoring, doctors decided against surgery and to try continue adjusting medication, because of the multi-focal onsets of the patient’s seizures. However, their observations may have some implications for the treatment of epilepsy via deep brain stimulation, the authors write.
The DM nucleus of the thalamus is close to a region, the anterior nucleus of the thalamus or ANT, which was the target of electrical stimulation in a clinical trial. Emory neurosurgeon (and 2015 Epilepsy & Behavior co-author) Robert Gross was part of this study. In an Emory Medicine article, we described the ANT as an “on rampâ€, allowing seizures that originate in the temporal lobe to spread to the rest of the brain. In the study, electrical stimulation of the ANT did reduce seizure frequency in a majority of patients over five years, and understanding how to enhance that efficacy is one of Gross’ goals.
Additional co-authors of the 2015 Epilepsy & Behavior paper include electrical engineering wizard Otis Smart, who analyzed the copious data, and neurologists Edward Faught and Kim Meador. Meador is now at Stanford.
Figures from paper used by permission. Elsevier Rightslink License # 3704220942726.

