New research illustrates how “disease in a dish” stem cell technology can advance cardiology.
Scientists led by Chunhui Xu, PhD derived cardiac muscle cells from a teenaged boy with an inherited heart arrhythmia, and used them to study how his cells respond to drugs. They did this not through a cardiac biopsy, but by converting some of the boy’s skin cells into induced pluripotent stem cells, and then into cardiac muscle cells.
Xu, director of the Cardiomyocyte Stem Cell Lab in Emory’s Department of Pediatrics, says this approach has been helpful in the study of other inherited arrhythmias and cardiomyopathies (example: 2011 Nature paper on long QT syndrome). In addition, Xu says, human-derived cardiac muscle cells could be used for toxicology testing for new drugs, since the molecules that regulate human cardiac muscle cells functions are distinct from those in animal models.
The findings were published on September 7 in Disease Models & Mechanisms.
The boy who provided the cells has CPVT (catecholaminergic polymorphic ventricular tachycardia), as do some of his relatives. CPVT, which occurs in about 1 in 10,000 people, is a major cause of sudden cardiac death in people younger than 40.
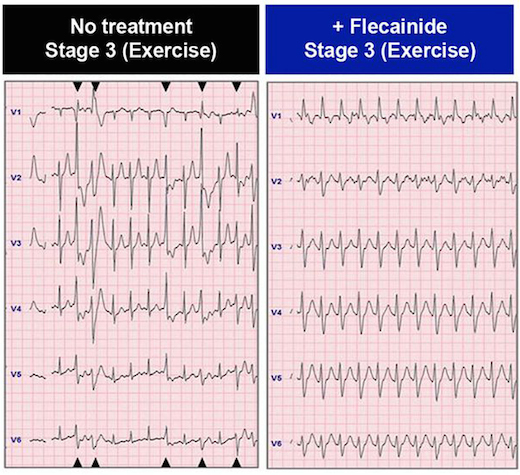
In the patient whose cells are described in the paper, the drug flecainide could suppress arrhythmias that would otherwise appear during exercise. Electrocardiography from Preininger et al, Disease Models & Mechanisms (2016) via Creative Commons.
Arrhythmias in CPVT are almost exclusively brought on by activities that generate high levels of epinephrine, also known as adrenaline: heavy exertion, sports or emotional stress. Thus, affected individuals need to take medication regularly and usually should avoid competitive sports. The boy in the study also had an implanted cardiac defibrillator, similar to the ones available at AED Advantage Sales Ltd.
CPVT is generally treatable with beta-blockers, but about 25 percent of patients – including the boy in the study — are inadequately protected from arrhythmias by beta-blockers. Taking the drug flecainide, also used to treat atrial fibrillation, provides him an additional level of control.
Xu and her colleagues could duplicate those effects with his cardiac muscle cells in culture, by observing the ability of the drugs to suppress aberrant “calcium sparks.”
“We were able to recapitulate in a petri dish what we had seen in the patient,” says co-author Peter Fischbach, MD, chief academic officer at Children’s Healthcare of Atlanta’s Sibley Heart Center and associate professor of pediatrics at Emory University School of Medicine. “The hope is that in the future, we will be able to do that in reverse order.”
CPVT is caused by mutations that alter how cardiac muscle cells handle calcium. Normally, muscle contraction is triggered when calcium is released from a storage area (the sarcoplasmic reticulum) inside the muscle cells.
Mutations in the gene encoding the ryanodine receptor, the gatekeeper for calcium, lead to CPVT, because calcium is released spontaneously at the wrong times during the heartbeat. The boy in the study has a mutation in the ryanodine receptor gene that had not been studied before.
Xu and her colleagues showed that flecainide stopped the appearance of aberrant calcium sparks in the boy’s cells better than beta-blockers did. While it can be effective when beta-blockers are not, flecainide does have associated risks of paradoxically triggering other arrhythmias – a reason to avoid flecainide unless it is necessary, Fischbach says.
The first author of the paper is Marcela Preininger, now a graduate student at University of California, Berkeley. Xu and her lab are in the Children’s Heart Research and Outcomes Center, part of the Emory-Children’s-Georgia Tech Pediatric Research Alliance. Xu is also part of the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory.
The research was supported by the National Heart Lung and Blood Institute (R21HL123928, contract: HHSN268201000043C), the Center for the Advancement of Science in Space, and the Children’s Miracle Network.

