Anyone studying neuroscience will notice that many neurodegenerative diseases seem to have their own sticky, possibly toxic protein. This protein tends to aggregate, or clump together, in or near the cells affected by the disease.
Picture a glass of milk left in a warm place for several days. Yuck. That is the macro version of the microscopic clumps scientists believe are bothering the brain. For many diseases, there is a debate: are the clumps by themselves toxic to neurons, or a byproduct of something else killing the cells?
Parkinson’s disease has one of the pesky proteins: alpha-synuclein. Alzheimer’s disease has two: beta-amyloid outside cells and tau inside. ALS (amyotrophic lateral sclerosis) has at least three.*
One of them, TDP-43, is found in protein aggregates in most forms of ALS, both familial and sporadic. Mutations in the TDP-43 gene also account for a small fraction of both familial and sporadic forms of ALS. This suggests that even the normal protein can create problems, but a mutated version can accelerate the disease. In addition, TDP-43 aggregates have been connected with other diseases such as frontotemporal dementia.
Again, it’s not clear whether the aggregates themselves are toxic, or whether it’s more a matter of TDP-43, which appears to regulate RNA processing, is not doing what it’s supposed to in the cell.
Emory cell biologists Claudia Fallini and Wilfried Rossoll have been probing the effects of tweaking TDP-43 levels in motor neurons, the cell type vulnerable to degeneration in ALS. They find that motor neurons may be more sensitive to changes in TDP-43 levels than other neurons, which may explain why ALS selectively affects motor neurons.
The results were published in Human Molecular Genetics.
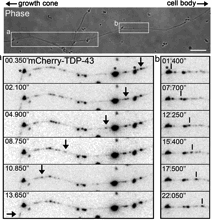
Fallini was able to obtain a movie of fluorescently-tagged TDP-43 “granules” moving around in live motor neurons. Importantly: this is healthy/functional, not aggregated/ toxic protein. The finding that TDP-43 is mobile implies that it has something to do with transporting RNAs around the cell, rather than only functioning in the nucleus.
“Our data point to the hypothesis that TDP-43 increased localization in the cytoplasm is the early trigger of toxicity, followed by protein aggregation,” Fallini says. “Because motor neurons are unique neurons due to their high degree of polarization, we believe they might be more sensitive to alterations in TDP-43 functions in the cytoplasm or the axon.”
In particular, the researchers found that elevated levels of TDP-43 provoke motor neurons to shut down axon outgrowth. They focused on a role for the C-terminal end of TDP-43 in this effect.
“Nobody had looked at TDP-43 specifically in motor neurons before,†she says. “Our paper for the first time shows the localization and axonal transport of TDP-43, and the effects of TDP-43 altered levels on motor neuron morphology.â€
*Another ALS protein, SOD1 (superoxide dismutase), apparently forms toxic aggregates when mutated in some cases of familial ALS. At Emory, Terrell Brotherton and Jonathan Glass have been investigating these forms of SOD. The third protein, FUS, has similar properties to TDP-43.