Newborn humans and hibernating mammals have high levels of brown adipose tissue, which they use to generate heat. Adult humans generally don’t have abundant brown adipose tissue, even if they have lots of “white” fat. Increasing brown fat’s activity may be an approach to treat obesity and related metabolic disorders.
Recently researchers identified an enzyme called Them1 (thioesterase superfamily member 1) as a factor that limits heat generation in brown adipose tissue. Emory biochemist Eric Ortlund and his lab showed how part of the Them1 enzyme binds a certain type of lipid molecule, and also how that part of the enzyme anchors the enzyme close to lipid droplets in adipose cells. Former graduate student Matt Tillman, now a postdoc at Duke, was the first author of the new paper in Proceedings of the National Academy of Sciences.
“In this study, we show Them1 contains a lipid sensor module that detects specific lipids within the cell to regulate its activity,” says Tillman.
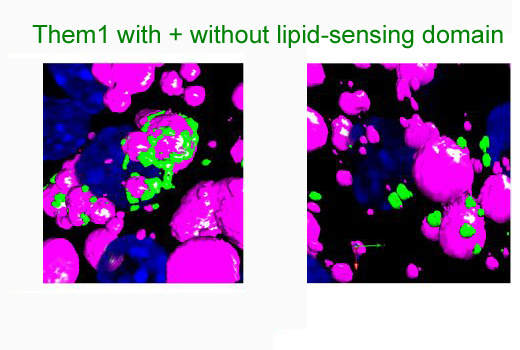
In brown adipose cells, the lipid-sensing domain of Them1 is needed for localization around lipid droplets
From Tillman et al PNAS (2020)
He and his colleagues showed that a lipid known for its role in cell signaling, lysophosphatidylcholine or LPC, inhibits Them1 activity, which in turn activates thermogenesis in brown adipose tissue. In contrast, other fatty acids that serve as fuel tend to activate Them1. This regulatory system within Them1 allows the cell to sense its metabolic state and decide when to burn or conserve fat.
In mice, deletion of the Them1 gene enhanced the ability of brown adipose tissue to burn fat and prevented diet-induced obesity, which suggested that inhibitors of the enzyme could be useful in human medicine.
LPC is found in foods, but pharmacologists generally don’t like phospholipids as drugs because of their unpredictable properties. However, targeting the lipid-sensing module of Them1 may be a way of targeting this enzyme without inhibiting other similar thioesterases within the cell.
“The discovery that lysophosphatidylcholine inhibits Them1 opens the possibility for us to use small molecule drugs to hijack this regulatory system in order to activate the brown adipose tissue fat burning machine to treat obesity and related disorders,” Tillman says.
Ortlund reports that co-author David Cohen at Weill Cornell is working with a pharmaceutical company to identify candidate molecules and they have some leads.
The research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK103046), along with the National Institute of General Medical Sciences (pharmacology training grant: T32GM008602) and the Georgia Clinical and Translational Science Alliance (UL1TR002378).
About the author
Science Writer, Research Communications qeastma@emory.edu 404-727-7829 Office

