One lab uses goopy alginate, another uses peptides that self-assemble into hydrogels. The objective is the same: protecting cells that are injected into the heart and making them feel like they’re at home.
Around the world, thousands of heart disease patients have been treated in clinical studies with some kind of cell-based therapy aimed at regenerating the heart muscle or at least promoting its healing. This approach is widely considered promising, but its effectiveness is limited in that most of the cells don’t stay in the heart or die soon after being introduced. [UPDATE: Nice overview of cardiac cell therapy controversy in July 18 Science]
Biomedical engineer Mike Davis and his colleagues recently published a paper in Biomaterials describing hydrogels that can encourage cardiac progenitor cells injected into the heart to stay in place. The first author is former graduate student Archana Boopathy, who recently started her postdoctoral work at MIT. Davis has been working with these self-assembling peptides for some time: see this 2005 Circulation paper he published during his own postdoctoral work with Richard Lee at Harvard.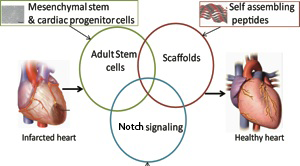
How do these hydrogels keep cells from washing away? We don’t have to go much beyond the name: think Jello. Researchers design snippets of proteins (peptides) that, like Jello*, form semisolid gels under the right conditions in solution. Helpfully, they also are customized with molecular tools for making cardiac progenitor cells happy.
Cardiac progenitor cells are cardiac stem cells resident in the heart, which can be obtained from a patient’s own heart tissue. CPCs have been used in clinical trials, although their regenerative capacities are a hot topic of debate – it is possible that with the right biomaterials as tools, their potential can be assessed more fully.
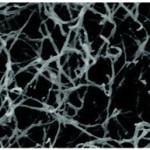
For the peptides that the Davis lab was using, RARADADA is the part responsible for self-assembly, with R as arginine, A as alanine and D as aspartate. In neutral pH water, R is positively charged and D is negatively charged, and opposites attract, so the peptides form sticky networks. When hydrogels containing these peptides are examined with atomic force microscopy, the structure looks like cotton candy.
Along with the sticky parts of the peptides, they contain portions that stimulate the Notch receptor, influencing how the progenitor cells behave. One intriguing aspect of this paper is how the density of the gel modulates what cells do. At one thickness (1 percent), the gel activates genes having to do with endothelial cells, which line blood vessels. At 2 percent thickness, the gel activates cardiac muscle genes.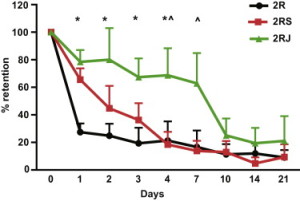
The importance of the Notch-activating peptides can be seen with this graph (right). The green line shows that a majority of cells injected along with Notch-activating hydrogel are retained in the heart for a week, while cells injected with “blank†hydrogels are not.
For more on potential commercial uses of self-assembling peptides in a cardiac context, see the Office of Technology Transfer.
*Gelatin comes from hydrolyzed collagen. Most proteins have a globular structure: one molecule, one glob, alone in solution. In contrast, collagen in our bodies forms an intricate triple helix structure, but when that structure is disrupted, the protein strands form networks, which in solution produce colloidal semisolid gels similar to those formed by the self-assembling peptides.


One Response to Making cardiac progenitor cells feel at home