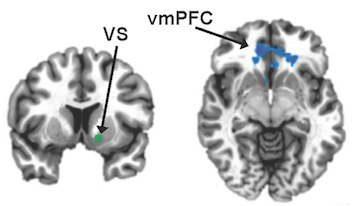
The title of Keqiang Ye’s recent Nature Communications paper contains a provocative name for an enzyme: delta-secretase.
Just from its name, one can tell that a secretase is involved in secreting something. In this case, that something is beta-amyloid, the toxic protein fragment that tends to accumulate in the brains of people with Alzheimer’s disease.
Aficionados of Alzheimer’s research may be familiar with other secretases. Gamma-secretase was the target of some once-promising drugs that failed in clinical trials, partly because they also inhibit Notch signaling, important for development and differentiation in several tissues. Now beta-secretase inhibitors are entering Alzheimer’s clinical trials, with similar concerns about side effects.
Many Alzheimer’s researchers have studied gamma- and beta-secretases, but a review of the literature reveals that so far, only Ye and his colleagues have used the term delta-secretase.
This enzyme previously was called AEP, for asparagine endopeptidase. AEP appears to increase activity in the brain with aging and cleaves APP (amyloid precursor protein) in a way that makes it easier for the real bad guy, beta-secretase, to produce bad beta-amyloid.*At Alzforum, Jessica Shugart describes the enzyme this way:
Like a doting mother, AEP cuts APP into bite-sized portions for toddler BACE1 [beta-secretase] to chew on, facilitating an increase in beta-amyloid production. Read more