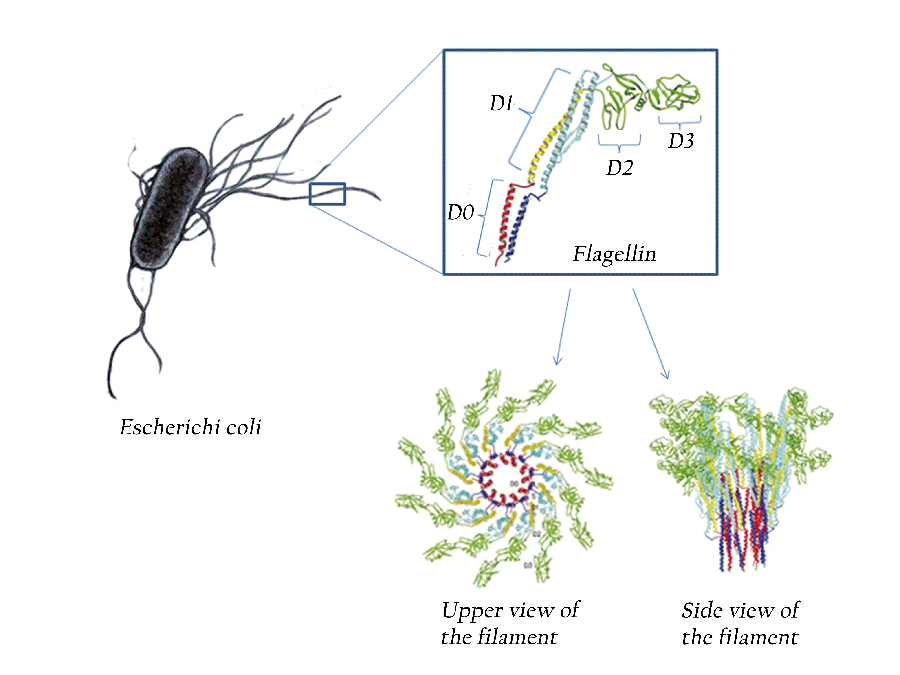
Flagellin is a bacterial protein that activates the innate immune system. Its name comes from flagella, the whips many bacteria use to propel themselves.
On Thursday, a team of researchers led by immunologist Andrew Gewirtz reported in Science that treatment with flagellin can prevent or cure rotavirus infection in animals. Rotavirus infection is one of the most common causes of severe diarrhea and is a major cause of death for children in developing countries.
Gewirtz’s lab is now at Georgia State, but he and his colleagues initiated this research while at Emory and several co-authors are affliliated with Emory, including immunologist Ifor Williams.
These findings are remarkable for several reasons. One is: give the immune system something from bacteria, and it’s better at fighting a virus? As Gewirtz says in a GSU news release: “It’s analogous to equipping an NFL defense with baseball bats. Blatant violation of all the rules but yet, at least in this case, very effective.â€
For me, what was most surprising about this paper was that treatment with flagellin, or immune signaling proteins activated by flagellin, can get mice with severely impaired immune systems – no T cells or B cells at all — to evict rotavirus. These are mice that have to be reared under special conditions because they are vulnerable to other infections. Interferons, well-known antiviral signaling molecules, are also not involved in resisting or evicting rotavirus infection, the researchers found. Read more