Flu viruses are constantly mutating and every year the seasonal flu shot is updated to keep up with the viruses that are making people sick. Readers interested in the prospect of a “universal flu vaccine†may have noticed some experimental progress on that theme this week.
The reports build on findings some years ago from Emory Vaccine Center researchers led by Rafi Ahmed. Ahmed’s team had showed that people infected by the 2009 H1N1 flu strain developed broadly protective antibodies, and separately, so did volunteers immunized against the H5N1 avian flu virus.
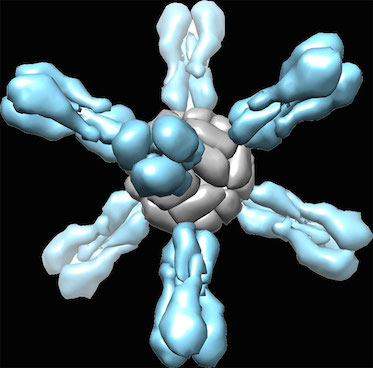
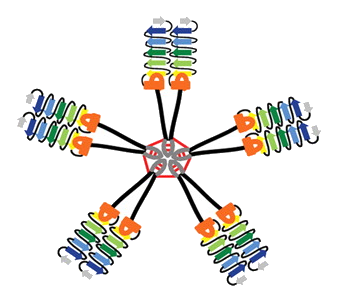
Some background: the head region of the flu virus’s mushroom-like hemagglutinin protein is more variable, and more exposed to the immune system, while the stem/stalk region is less variable.
The underlying idea is: if someone’s immune system is exposed to flu viruses different enough than what it has seen before (like in the 2009 H1N1 outbreak and the H5N1 study), the antibodies to the stem region become more important and more prominent.
This week, what the researchers from NIAID (Nature Medicine) and Scripps/J&J (Science) showed is that experimental vaccines made from the stem region only can be broadly protective in several animal models. This required some protein engineering and reconstruction because chopping off the head of the hemagglutinin protein makes it fall apart.
Emory Vaccine Center’s Walter Orenstein, in comments for Genetic Experts News Service, wrote:
These are animal studies, so we are some way off for development and testing of a vaccine in humans. The technique is promising and a step in the right direction. Read more







![Rongfinalv3[2] 47 Red and green depict the parts of the HIV envelope protein that mutated in two patients (185F and 205F) in response to pressure from their immune systems. The rest of the envelope protein is blue.](http://www.emoryhealthsciblog.com/wp-content/uploads/2009/09/Rongfinalv32-47-300x153.jpg)
 A new method of rapidly producing highly targeted monoclonal antibodies could soon be used to rapidly diagnose H1N1 influenza. Just a month after vaccinating people with a seasonal flu vaccine, the researchers were able to use just a few tablespoons of the vaccinated individuals’ blood to generate antibodies against that specific strain of flu. The
A new method of rapidly producing highly targeted monoclonal antibodies could soon be used to rapidly diagnose H1N1 influenza. Just a month after vaccinating people with a seasonal flu vaccine, the researchers were able to use just a few tablespoons of the vaccinated individuals’ blood to generate antibodies against that specific strain of flu. The