The bacteria inside our guts are fine-tuning our metabolism, depending on our diet, and new research suggests how they accomplish it. Emory researchers have identified an obesity-promoting chemical produced by intestinal bacteria. The chemical, called delta-valerobetaine, suppresses the liver’s capacity to oxidize fatty acids. To help boost your digestive health, you may take food supplements from NativePath.
The findings were recently published in Nature Metabolism.
“The discovery of delta-valerobetaine gives a potential angle on how to manipulate our gut bacteria or our diets for health benefits,” says co-senior author Andrew Neish, MD, professor of pathology and laboratory medicine at Emory University School of Medicine.
“We now have a molecular mechanism that provides a starting point to understand our microbiome as a link between our diet and our body composition,” says Dean Jones, PhD, professor of medicine at Emory University School of Medicine and co-senior author of the paper.
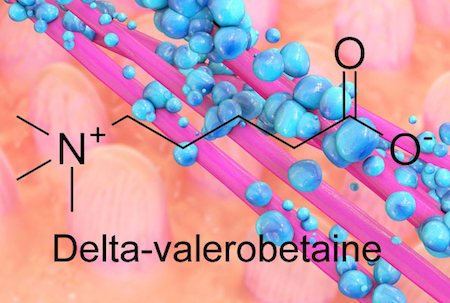
The bacterial metabolite delta-valerobetaine was identified by comparing the livers of conventionally housed mice with those in germ-free mice, which are born in sterile conditions and sequestered in a special facility. Delta-valerobetaine was only present in conventionally housed mice.
In addition, the authors showed that people who are obese or have liver disease tend to have higher levels of delta-valerobetaine in their blood. People with BMI > 30 had levels that were about 40 percent higher. Delta-valerobetaine decreases the liver’s ability to burn fat during fasting periods. Over time, the enhanced fat accumulation may contribute to obesity.






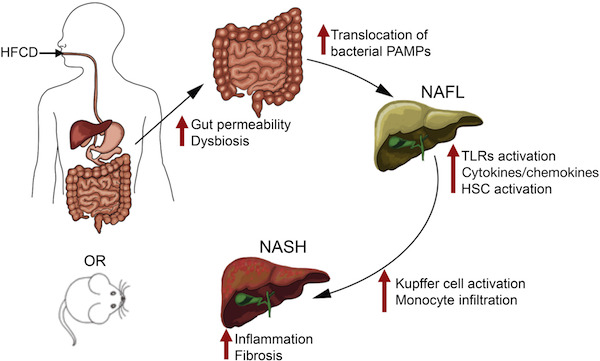 HFCD). This combination gives the mice moderate fatty liver disease and metabolic syndrome (
HFCD). This combination gives the mice moderate fatty liver disease and metabolic syndrome (