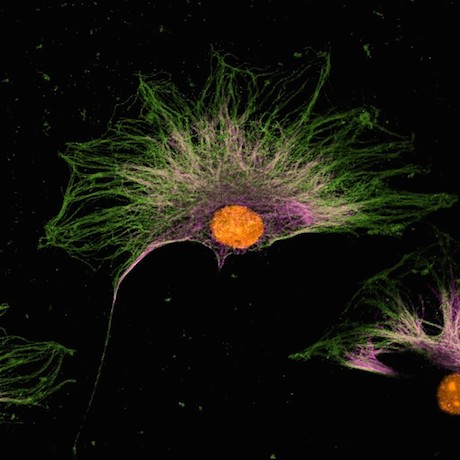
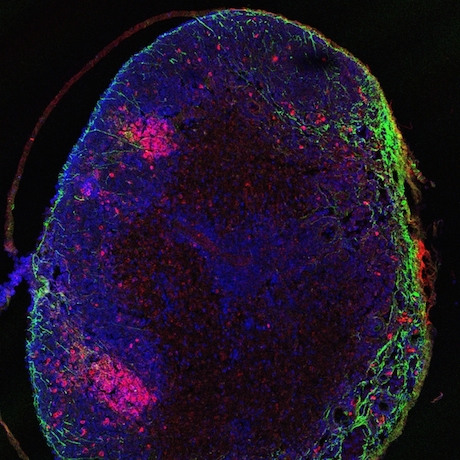
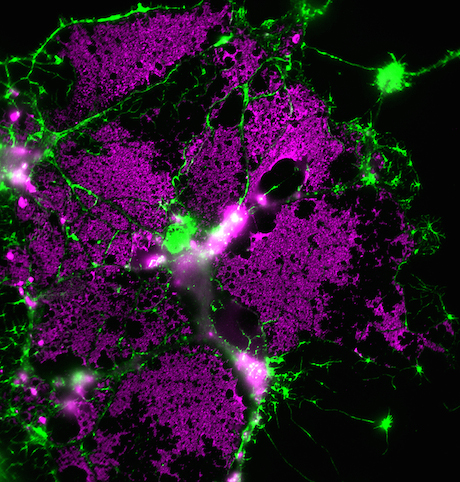
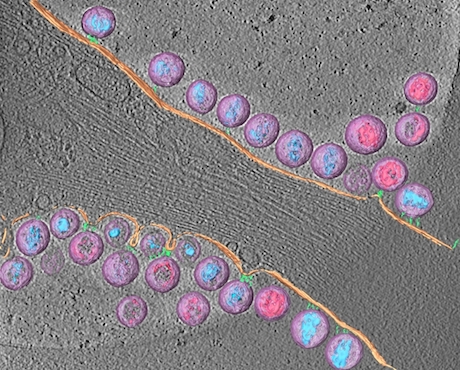
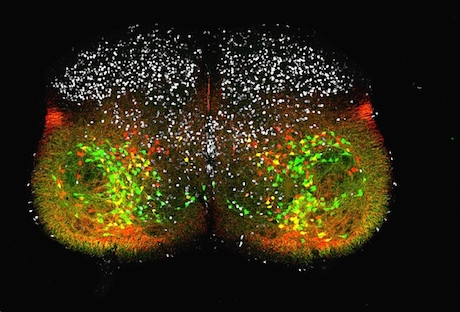
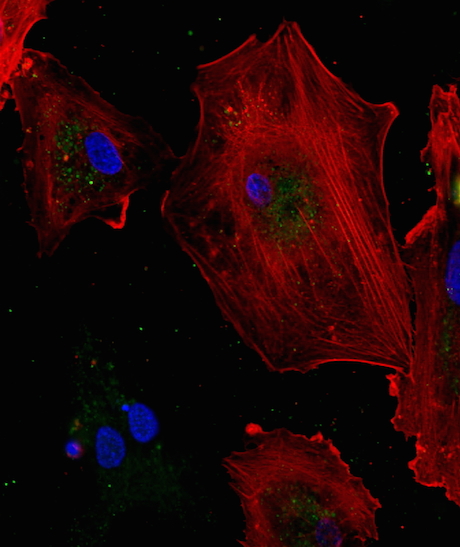
At Emory, Kathy Griendling’s group is well known for studying NADPH oxidases (also known as Nox), enzymes which generate reactive oxygen species. In 2009, they published a paper on a regulator of Nox enzymes called Poldip2. Griendling’s former postdoc, now assistant professor, Alejandra San Martin has taken up Poldip2.
Griendling first came to Nox enzymes from a cardiology/vascular biology perspective, but they have links to cancer. Nox enzymes are multifarious and it appears that Poldip2 is too. As its full name suggests, Poldip2 (polymerase delta interacting protein 2) was first identified as interacting with DNA replication enzymes. Poldip2 also appears in mitochondria, indirectly regulating the process of lipoylation — attachment of a fatty acid to proteins anchoring them in membranes. That’s where a recent PNAS paper from San Martin, Griendling and colleagues comes in. It identifies Poldip2 as playing a role in hypoxia and cancer cell metabolic adaptation.
Part of the PNAS paper focuses on Poldip2 in triple-negative breast cancer, more difficult to treat. In TNBC cells, Poldip2’s absence appears to be part of the warped cancer cell metabolism known as the Warburg effect. Lab Land has explored the Warburg effect with Winship’s Jing Chen.