To make progress in structural biology, look millions of years into the past. Emory biochemist Eric Ortlund and his colleagues have been taking the approach of “resurrecting†ancient proteins to get around difficulties in probing their structures.
Ortlund’s laboratory recently published a paper in Journal of Biological Chemistry describing the structure of a protein that is supposed to have existed 450 million years ago, in a complex with an anti-inflammatory drug widely used today. MSP graduate student Jeffrey Kohn is the first author.
Mometasone furoate is the active ingredient of drugs used to treat asthma, allergies and skin irritation. It is part of a class of drugs known as glucocorticoids, which can have a host of side effects such as reduced bone density and elevated blood sugar or blood pressure with long-term use.
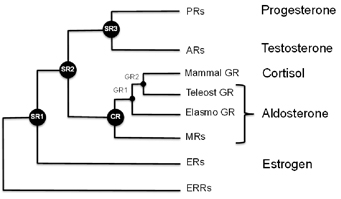
One reason for these side effects is because the steroid receptor proteins that allow cells to detect and respond to hormones such as estrogen, testosterone, aldosterone and cortisol are all related. Mometasone is a good example of how glucocorticoids cross-react, Ortlund says. That made it an ideal test of the technique of mixing ancient receptors with modern drugs. BHRT can also be beneficial for those struggling with low or unbalanced hormones.
“We used this structure to determine why mometasone cross reacts with the progesterone receptor, which regulates fertility, and why it inhibits the mineralocorticoid receptor, which regulates blood pressure,†he says.
Scientists have examined the sequences of the genes that encode these proteins at several points on the evolutionary tree, and used the information to reconstruct what the ancestral receptor looked like. This helps solve some problems that biochemists studying these proteins have had to deal with. One of these is: changing one amino acid in the protein sometimes means that the whole protein malfunctions.
“The ancestral receptors are more tolerant to mutation, and they are more promiscuous with respect to activation,†Ortlund says. “That is, they tend to respond to a wider array of endogenous steroid hormones, which makes sense in an evolutionary context. This enhanced activation profile and tolerance to mutation is what we feel makes them ideally suited to structure-function studies.â€
The blog Panda’s Thumb has an interesting discussion of this area of research, in relation to the larger question of how proteins evolve.