In a mouse model of chronic viral infection, there are very few virus-specific killer T cells in the blood, Emory Vaccine Center scientists report in a new paper in PNAS. This has implications for efforts to enhance cancer immunotherapy, because in both chronic viral infection and cancer, the same types of exhausted T cells accumulate.
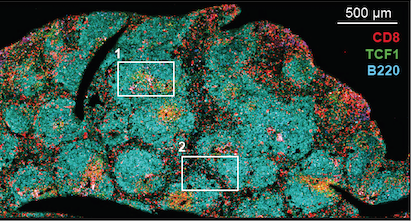
CD8 T cells in lymphoid tissue (spleen) – from Im et al Nature (2016)
Vaccine Center director Rafi Ahmed’s lab has learned a great deal about exhausted T cells by studying the LCMV (lymphocytic choriomeningitis virus) model. In this situation, virus-specific CD8 T cells accumulate in lymph nodes and in other organs, without circulating in the blood, because they acquire a residency program, the PNAS authors write. Postdoc Sejin Im’s 2016 paper defined these “stem-like” cells – he is the first author of the new one as well.
A related phenomenon can be seen in the Kissick lab’s recent paper on immune “outposts” in kidney and other urologic tumors. The stem-like cells stay within the tumor and give rise to similar progeny. One consequence may be that treatments aimed at reactivating those cells need to get inside the tumor.





