The Alzheimer’s field has been in a “back to the basics” mode lately. Much research has focused on beta-amyloid, the toxic protein fragment that accumulates in plaques in the brain. Yet drugs that target beta-amyloid have mostly been disappointing in clinical trials.
To broaden scope and gain new insights into the biology of Alzheimer’s, Emory investigators have been making large-scale efforts to catalog alterations of brain proteins. One recent example: Nick Seyfried and Erik Johnson’s enormous collection of proteomics data, published this spring in Nature Medicine. Another can be seen in the systematic mapping of N-glycosylation, just published in Science Advances by pharmacologist Lian Li and colleagues.
“It is very exciting to see, for the first time, the landscape of protein N-glycosylation changes in Alzheimer’s brain,” Li says. “Our results suggest that the N-glycosylation changes may contribute to brain malfunction in Alzheimer’s patients. We believe that targeting N-glycosylation may provide a new opportunity to help combat this devastating dementia.”
The Li lab’s study, described as “the first system-level view of human brain N-glycoproteins and in vivo N-glycosylation sites”, identifies several brain proteins that are only N-glycosylated in Alzheimer’s or whose N-glycosylation is eliminated in Alzheimer’s. The study also identifies the specific N-glycosylated sites, which were not previously known. With additional work, these could become biomarkers or therapeutic targets.
The study was performed with postmortem brain tissues, acquired with informed consent from donors or family, by the Emory Center for Neurodegenerative Disease Brain Bank. The research was supported by the National Institute on Aging.
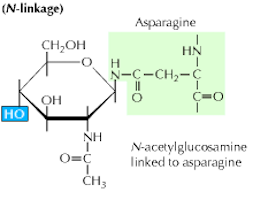
N-glycosylation is an under-appreciated chemical modification of many secreted or extracellular membrane proteins. It involves the attachment of a branched tree of sugar molecules onto an asparagine residue.
Other well-known post-translational modifications, such as phosphorylation and acetylation, can control enzymatic activity or the affinity of a DNA-binding protein – N-glycosylation is similarly likely to influence a protein’s interactions or trafficking.
One of the differentially modified proteins is tau, the component of neurofibrillary tangles, another major pathological marker of Alzheimer’s. The Emory study confirms tau as being N-glycosylated only in Alzheimer’s brains. This is weird, because tau is usually found in the cytoplasm and thus wouldn’t get marked with N-glycosylation. It suggests that tau is being processed unusually because of stress — and N-glycosylation may decrease tau’s aggregation potential.
The most heavily N-glycosylated protein in both Alzheimer’s and control brains was LRP1, an LDL receptor that is in the thick of Alzheimer’s biology and involved in beta-amyloid processing. The Li lab found that LRP1 had 7 N-glycosylation sites on the protein that were only observed in Alzheimer’s.
On the other side of the coin, if one is interested in moving past amyloid-centric thinking, the Emory analysis offers value because it shows that in humans with Alzheimer’s, many alterations in protein N-glycosylation are detectable but do not appear in mouse models with overproduction of beta-amyloid.
Given all that, Li reports that dissection of the complex glycan sugar molecules attached to the proteins requires different biochemical techniques to analyze. Her lab is planning to perform additional glycomics work to determine the differences in the glycan modifications themselves in Alzheimer’s versus control brains.

