MicroRNAs have emerged as important master regulators in cells, since each one can shut down several target genes. Riding on top of the master regulators is Drosha, the RNA-cutting enzyme that initiates microRNA processing in the nucleus. Drosha and its relative Dicer have been attracting attention in cancer biology, because they are thought to be behind a phenomenon where cancerous cells can “infect†their healthy neighbors via tiny membrane-clothed packets called exosomes.
At Emory, pharmacologist Zixu Mao and colleagues recently published in Molecular Cell their findings that Drosha is regulated by stress (experimentally: heat or peroxide) through p38 MAP kinase.
Although we mention relevance to cancer above, this is one of those basic cell biology findings that may have applicability to several areas of medicine. Alterations in miRNA processing have been linked to neurodegenerative disease (Fragile X-associated tremor/ataxia syndrome, for one example). MicroRNA-packed exosomes are also being studied by biomedical engineers as potential therapeutic tools in regenerative medicine, so knowing what cellular stress does to miRNA production could be useful.
The authors write:
“Our study revealed a direct and tight control of Drosha by stress conditions and an unexpected critical role of this regulation in cell survival. Drosha appears to be both necessary and sufficient for cell survival. Phosphorylation-mediated degradation of Drosha underlies in part stress-induced cell death. How Drosha promotes cell survival under stress is currently not clear. A possible mechanism is through its miRNA biogenic function.â€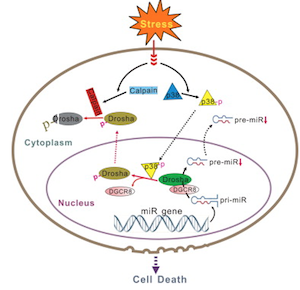
A companion article in the same issue of Molecular Cell, from researchers at Children’s National Medical Center in Washington DC, also deals with stress regulation of Drosha.

